MacroArray ALLERGY XPLORER Macro Array Diagnostics
Specifications
- Product Name: Basic UDI-DI 91201229202JQ
- Reference Numbers: REF 02-2001-01, 02-5001-01
- Intended Use: Detection of allergen-specific IgE (sIgE) quantitatively and total IgE (tIgE) semi-quantitatively
- Users: Trained laboratory personnel and medical professionals in a medical laboratory
- Storage: Kit reagents are stable for 6 months after opening
Product Usage Instructions
Principle of the Procedure
The product detects allergen-specific IgE quantitatively and total IgE semi-quantitatively.
Shipment and Storage
Ensure kit reagents are stored as indicated and are used within 6 months of opening.
Waste Disposal:
Follow proper waste disposal procedures as per regulations.
Kit Components
Refer to the user manual for detailed information on kit components.
Required Equipment
Manual Analysis: Ensure you have the necessary equipment provided by the manufacturer.
Automatic Analysis: Use MAX device, Washing Solution, Stop Solution, RAPTOR SERVER Analysis Software, and a PC/Laptop. Follow maintenance instructions carefully.
Handling of Arrays
Follow the instructions provided for handling arrays carefully to ensure accurate results.
Warnings and Precautions
- Wear appropriate protective gear like hand and eye protection and lab coats.
- Handle reagents and samples following good laboratory practices.
- Treat all human source materials as potentially infectious and handle them with care.
FAQ
- Q: How long are kit reagents stable for?
A: Kit reagents are stable for 6 months after opening when stored under the indicated conditions. - Q: Who can use this product?
A: This product is intended for use by trained laboratory personnel and medical professionals in a medical laboratory setting.
www.madx.com
ALLERGY XPLORER (ALEX²) INSTRUCTION FOR USE
DESCRIPTION
The Allergy Xplorer (ALEX²) is an Enzyme-Linked Immunosorbent Assay (ELISA) – based in-vitro diagnostic tests for the quantitative measurement of allergen-specific IgE (sIgE).
This Instruction for Use is applicable for the following products:
| Basic UDI-DI | REF | Product |
| 91201229202JQ | 02-2001-01 | ALEX² for 20 Analyses |
| 02-5001-01 | ALEX² for 50 Analyses |
INTENDED PURPOSE
The ALEX² Allergy Xplorer is a test kit used for in-vitro examination of human serum or plasma (exception EDTA-plasma) to provide information to aid the diagnosis of patients suffering from IgE-mediated diseases in conjunction with other clinical findings or diagnostic test results.
The IVD medical device detects allergen-specific IgE (sIgE) quantitatively and total IgE (tIgE) semi-quantitatively. The product is used by trained laboratory personnel and medical professionals in a medical laboratory.
SUMMARY AND EXPLANATION OF THE TEST
Allergic reactions are immediate type I hypersensitivity reactions and are mediated by antibodies belonging to the IgE class of immunoglobulins. After exposure to specific allergens, IgE-mediated release of histamine and other mediators from mast cells and basophils results in clinical manifestations such as asthma, allergic rhino-conjunctivitis, atopic eczema, and gastrointestinal symptoms [1]. Therefore, a detailed sensitization pattern to specific allergens assists in the evaluation of allergic patients [2-6]. There is no restriction on the test population. When developing IgE assays, age and sex are typically not considered as critical factors because IgE levels, which are measured in these assays, do not significantly vary based on these demographics.
All major type I allergen sources are covered by ALEX². A complete list of ALEX² allergen extracts and molecular allergens can be found at the bottom of this instruction.
Important information for the user!
For the correct use of ALEX², it is necessary for the user to carefully read and follow these instructions for use. The manufacturer assumes no liability for any use of this test system which is not described in this document or for modifications by the user of the test system.
Attention: The kit variant 02-2001-01 of the ALEX² test (20 Arrays) is exclusively intended for manual processing. To use this ALEX² kit variant with the automated MAX 9k, the Washing Solution (REF 00-5003-01) and the Stop Solution (REF 00-5007-01) need to be ordered separately. All further product information can be found in the corresponding instructions for use: https://www.madx.com/extras.
The ALEX² kit variant 02-5001-01 (50 arrays) can be used for automated processing with the MAX 9k (REF 17-0000-01) as well as the MAX 45k (REF 16-0000-01) device.
PRINCIPLE OF THE PROCEDURE
ALEX² is an immunoassay test based on Enzyme-Linked Immunosorbent Assay (ELISA). Allergen extracts or molecular allergens, which are coupled to nanoparticles, are deposited in a systematic fashion onto a solid phase forming a macroscopic array. First, the particle-bound allergens react with specific IgE that is present in the patient’s sample. After incubation, non-specific IgE is washed off. The procedure continues by adding an enzyme-labelled anti-human IgE detection antibody which forms a complex with the particle-bound specific IgE. After a second washing step, substrate is added which is converted to an insoluble, colored precipitate by the antibody-bound enzyme. Finally, the enzyme-substrate reaction is stopped by adding a blocking reagent. The amount of precipitate is proportional to the concentration of specific IgE in the patient’s sample. The lab test procedure is followed by image acquisition and analysis using either the manual system (ImageXplorer) or the automated system (MAX 45k or MAX 9k). The test results are analyzed with RAPTOR SERVER Analysis Software and reported in IgE response units (kUA/l). Total IgE results are also reported in IgE response units (kU/l). RAPTOR SERVER is available in version 1, for the full four-digit version number please refer to the RAPTOR SERVER imprint available at www.raptor-server.com/imprint.
SHIPMENT AND STORAGE
The shipment of ALEX² takes place at ambient temperature conditions. Nevertheless, the kit must be stored immediately upon delivery at 2-8°C. Stored correctly, ALEX² and its components can be used until the indicated expiration date.
Kit reagents are stable for 6 months after opening (at the indicated storage conditions).
WASTE DISPOSAL
Dispose the used ALEX² cartridge and unused kit components with laboratory chemical waste. Follow all national, state, and local regulations regarding disposal.
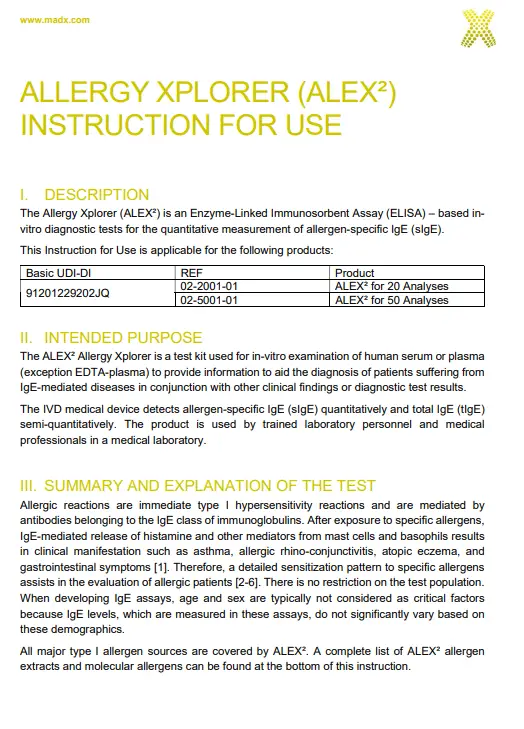
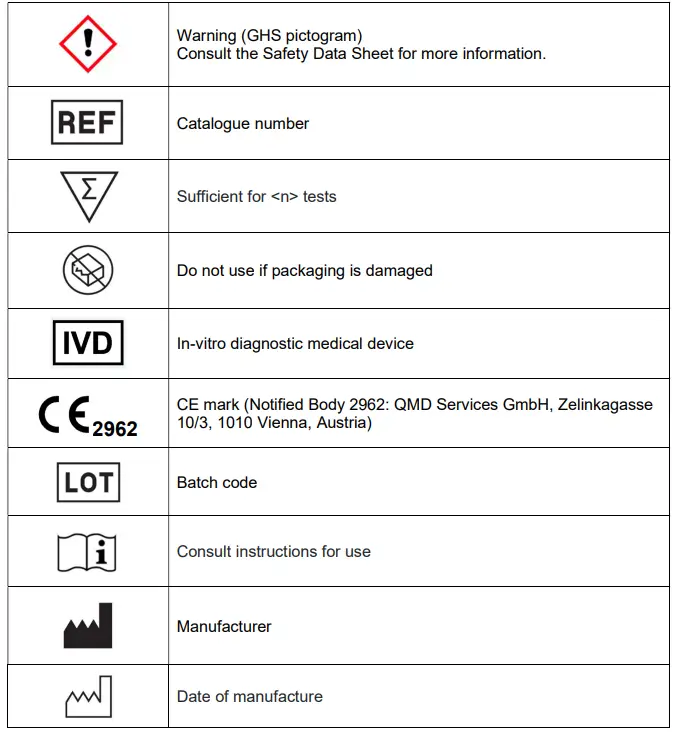
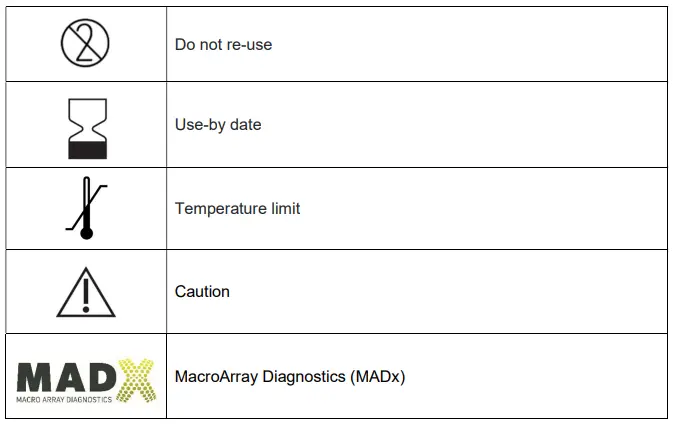
GLOSSARY OF SYMBOLS
KIT COMPONENTS
Each component (reagent) is stable until the date stated on each individual component’s label. It is not recommended to pool any reagents from different kit lots. For a list of allergen extracts and molecular allergens immobilized on the ALEX² array, please contact support@madx.com.
| Kit Components REF 02-2001-01 | Content | Properties |
| ALEX² Cartridge | 2 Blisters à 10 ALEX² for 20 analyses in total.
Calibration via master curve available via RAPTOR SERVER Analysis Software. |
Ready for use. Store at 2-8°C until expiry date. |
| ALEX² Sample Diluent | 1 bottle à 9 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C, includes CCD inhibitor. |
| Washing Solution | 2 bottle à 50 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. |
| Kit Components REF 02-2001-01 | Content | Properties |
| ALEX² Detection Antibody | 1 bottle à 11 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. |
| ALEX² Substrate Solution | 1 bottle à 11 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. |
| (ALEX²) Stop Solution | 1 bottle à 2.4 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. May appear as a turbid solution after prolonged storage. This has no effect on results. |
| Kit Components REF 02-5001-01 | Content | Properties |
| ALEX² Cartridge | 5 Blisters à 10 ALEX² for 50 analyses in total.
Calibration via master curve available via RAPTOR SERVER Analysis Software. |
Ready for use. Store at 2-8°C until expiry date. |
| ALEX² Sample Diluent | 1 bottle à 30 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C, includes CCD inhibitor. |
| Washing Solution | 4 x conc. 1 bottle à 250 ml | Store at 2-8°C until expiry date. Dilute 1 to 4 with demineralized water before use. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. |
| ALEX² Detection Antibody | 1 bottle à 30 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. |
| Kit Components REF 02-5001-01 | Content | Properties |
| ALEX² Substrate Solution | 1 bottle à 30 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is
stable for 6 months at 2-8°C. |
| (ALEX²) Stop Solution | 1 bottle à 10 ml | Ready for use. Store at 2-8°C until expiry date. Allow reagent to reach room temperature before use. Opened reagent is stable for 6 months at 2-8°C. May appear as a turbid solution after prolonged storage. This has no effect on results. |
REQUIRED EQUIPMENT FOR PROCESSING AND ANALYSING
Manual Analysis
- ImageXplorer
- Arrayholder (optional)
- Lab Rocker (inclination angle 8°, required speed 8 rpm)
- Incubation chamber (WxDxH – 35x25x2 cm)
- RAPTOR SERVER Analysis Software
- PC/Laptop
Required equipment, not provided by MADx:
- Demineralized Water
- Pipettes & tips (100 µl & 100 – 1000 µl)
Automatic Analysis:
- MAX device (MAX 45k or MAX 9k)
- Washing Solution (REF 00-5003-01)
- Stop Solution (REF 00-5007-01)
- RAPTOR SERVER Analysis Software
- PC/Laptop
Maintenance services according to manufacturer’s instructions.
HANDLING OF ARRAYS
Do not touch the array surface. Any surface defects caused by blunt or sharp objects can interfere with the correct readout of the results. Do not acquire ALEX² images before array is completely dry (dry at room temperature).
WARNINGS AND PRECAUTIONS
- It is recommended to wear hand and eye protection as well as lab coats and follow good laboratory practices when preparing and handling reagents and samples.
- In accordance with good laboratory practice, all human source material should be considered potentially infectious and handled with the same precautions as patient samples.
- ALEX² Sample Diluent and Washing Solution contain sodium azide (<0.1%) as a preservative and must be handled with care. Safety data sheet is available upon request.
- The (ALEX²) Stop Solution contains Ethylenediaminetetraacetic acid (EDTA)-Solution and must be handled with care. Safety data sheet is available upon request.
- For in-vitro diagnostic use only. Not for internal or external use in humans or animals.
- Only personnel trained in laboratory practice should use this kit.
- Upon arrival, check the kit components for damage. If one of the components is damaged (e.g. buffer bottles), contact MADx (support@madx.com) or your local distributor. Do not use damaged kit components, as their use may lead to poor kit performance.
- Do not use reagents beyond their expiry dates.
- Do not mix reagents from different batches.
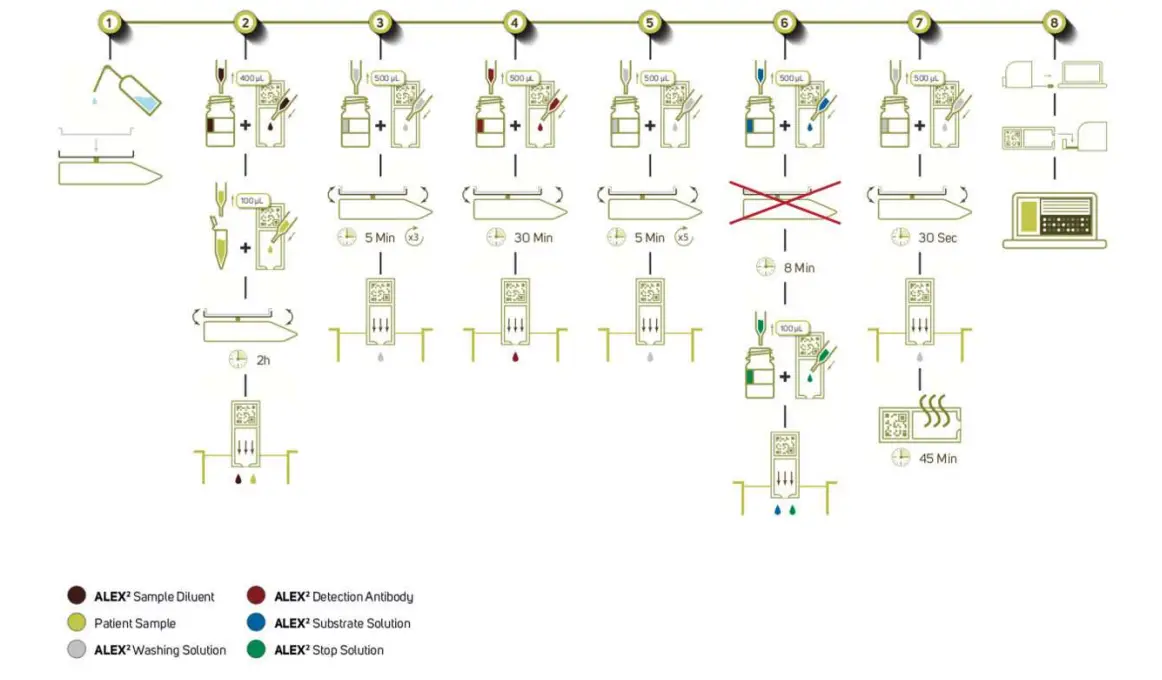
ELISA PROCEDURE
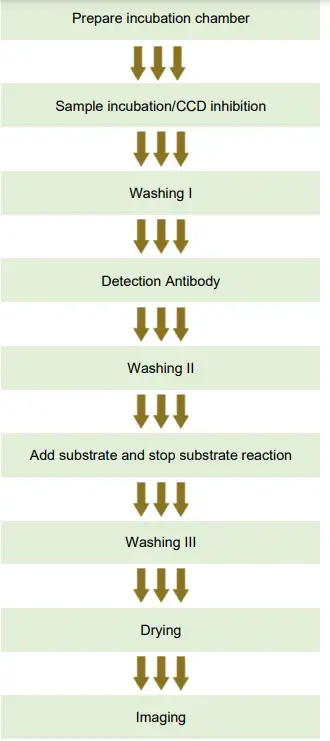
Preparation
Preparation of samples: Serum or plasma (heparin, citrate, no EDTA) samples from capillary or venous blood can be used. Blood samples can be collected using standard procedures. Store samples at 2–8°C for up to one week. Keep serum and plasma samples at -20°C for prolonged storage. Shipment of serum/plasma samples at room temperature is applicable. Always allow samples to reach room temperature before use.
Preparation of Washing Solution (only for REF 02-5001-01 and REF 00-5003-01 when used with MAX device): Pour the content of 1 vial of Washing Solution into the washing container of the instrument. Fill demineralized water up to the red mark and carefully mix the container several times without generating foam. The opened reagent is stable for 6 months at 2-8°C.
Incubation chamber: Close lid for all assay steps to prevent drop in humidity.
Parameters of Procedure:
- 100 µl sample + 400 µl ALEX² Sample Diluent
- 500 µl ALEX² Detection Antibody
- 500 µl ALEX² Substrate Solution
- 100 µl (ALEX²) Stop Solution
- 4500 µl Washing Solution
Assay time is approximately 3 h 30 min (without drying of processed array).
It is not recommended to run more assays than can be pipetted in 8 min. All incubations are performed at room temperature, 20-26°C.
All reagents are to be used at room temperature (20-26°C). The assay must not be performed in direct sunlight.
Prepare incubation chamber
Open incubation chamber and place paper towels on bottom part. Soak paper towels with demineralized water until no dry parts of the paper towels are visible.
Sample incubation/CCD inhibition
Take out the needed number of ALEX² cartridges and place them into the array holder(s). Add 400 μl of ALEX² Sample Diluent to each cartridge. Add 100 μl patient sample to the cartridges. Ensure that the resulting solution is spread evenly. Place the cartridges in the prepared incubation chamber and put the incubation chamber with the cartridges on the lab rocker so that the cartridges rock along the long side of the cartridge. Start the serum incubation with 8 rpm for 2 hours. Close incubation chamber before starting the lab rocker. After 2 hours, discharge the samples into a collection container. Carefully wipe off droplets from the cartridge using a paper towel.
Avoid touching the array surface with the paper towel! Avoid any carry over or cross-contamination of samples between individual ALEX² cartridges!
Optional or positive Hom s LF (CCD marker): with the standard CCD antibody inhibition protocol (as described in paragraph 2: sample incubation/CCD inhibition) the CCD inhibition efficiency is 85%. If a higher rate of inhibition efficiency is required, prepare a 1 ml sample tube, add 400 μl ALEX² Sample Diluent and 100 μl serum. Incubate for 30 minutes (non-shaking) and then proceed with the usual assay procedure.
Note: The extra CCD inhibition step leads in many cases to an inhibition rate for CCD antibodies of above 95%.
1a. Washing I
Add 500 μl Washing Solution to each cartridge and incubate on the lab rocker (at 8 rpm) for 5 minutes. Discharge the Washing Solution into a collection container and vigorously tap the cartridges on a stack of dry paper towels. Carefully wipe off remaining droplets from the cartridges using a paper towel.
Repeat this step 2 more times.
Add detection antibody
Add 500 µl of ALEX² Detection Antibody to each cartridge.
Make sure that the complete array surface is covered by the ALEX² Detection Antibody solution.
Place the cartridges into the incubation chamber on the lab rocker and incubate at 8 rpm for 30 minutes. Discharge the Detection Antibody solution into a collection container and vigorously tap the cartridges on a stack of dry paper towels. Carefully wipe off remaining droplets from the cartridges using a paper towel.
2a. Washing II
Add 500 μl Washing Solution to each cartridge and incubate on the lab rocker at 8 rpm for 5 minutes. Discharge the Washing Solution into a collection container and vigorously tap the cartridges on a stack of dry paper towels. Carefully wipe off remaining droplets from the cartridges using a paper towel.
Repeat this step 4 more times.
3+4. Add ALEX² Substrate Solution and stop substrate reaction
Add 500 μl of ALEX² Substrate Solution to each cartridge. Start a timer with filling the first cartridge and proceed with the filling of the remaining cartridges. Make sure that the complete array surface is covered by the Substrate Solution and incubate the arrays for exactly 8 minutes without shaking (lab rocker at 0 rpm and in horizontal position).
After exactly 8 minutes, add 100 μl of the (ALEX²) Stop Solution to all cartridges, starting with the first cartridge to assure that all arrays are incubated for the same time with the ALEX² Substrate Solution. Carefully agitate to evenly distribute the (ALEX²) Stop Solution in the array cartridges, after the (ALEX²) Stop Solution was pipetted onto all arrays. Afterwards discharge the (ALEX²) Substrate/Stop Solution from the cartridges and vigorously tap the cartridges on a stack of dry paper towels. Carefully wipe off any remaining droplets from the cartridges using a paper towel.
The Lab Rocker must NOT SHAKE during substrate incubation!
4a. Washing III
Add 500 μl Washing Solution to each cartridge and incubate on the lab rocker at 8 rpm for 30 seconds. Discharge the Washing Solution into a collection container and vigorously tap the cartridges on a stack of dry paper towels. Carefully wipe off any remaining droplets from the cartridges using a paper towel.
Image analysis
After finishing the assay procedure, air dry the arrays at room temperature until they are completely dry (can take up to 45 min).
The complete drying is essential for the sensitivity of the test. Only completely dried arrays provide an optimal signal to noise ratio.
Finally, the dried arrays are scanned with the ImageXplorer or a MAX device and analyzed with RAPTOR SERVER Analysis software (see details in the RAPTOR SERVER software handbook). The RAPTOR SERVER Analysis Software is only verified in combination with the ImageXplorer instrument and the MAX devices, therefore MADx does not take any responsibilities for results, which have been obtained with any other image capture device (like scanners).
Assay Calibration
The ALEX² master calibration curve was established by reference testing against serum preparations with specific IgE against different antigens covering the intended measuring range. Lot specific calibration parameters are provided by the RAPTOR SERVER Analysis Software. ALEX² sIgE test results are expressed as kUA/l. Total IgE results are semi-quantitative and calculated from an anti-IgE measurement with lot-specific calibration factors, which are provided by the RAPTOR SERVER Analysis Software and selected according to the lot-specific QR-codes.
Curve parameters for each lot are adjusted by an in-house reference testing system, against serum preparations tested on ImmunoCAP (Thermo Fisher Scientific) for specific IgE against several allergens. The ALEX² results are therefore indirectly traceable against the WHO reference preparation 11/234 for total IgE.
Systematic variations in signal levels between lots are normalized by heterologous calibration against an IgE reference curve. A correction factor is used to systematically adjust for lot-specific measurement deviations.
Measuring Range
Specific IgE: 0.3-50 kUA/l quantitative
Total IgE: 20-2500 kU/l semi-quantitative
QUALITY CONTROL
Record keeping for each assay
According to good laboratory practice it is recommended to record the lot numbers of all reagents used.
Control Specimens
According to good laboratory practice it is recommended that quality control samples are included within defined intervals. Reference values for certain commercially available control sera can be provided by MADx upon request.
DATA ANALYSIS
For the image analysis of processed arrays, the ImageXplorer or a MAX device is to be used. ALEX² images are automatically analyzed using RAPTOR SERVER Analysis Software and a report is generated summarizing the results for the user.
RESULTS
ALEX² is a quantitative ELISA test for specific IgE and semi-quantitative method for total IgE. Allergen-specific IgE antibodies are expressed as IgE response units (kUA/l), total IgE results as kU/l. RAPTOR SERVER Analysis Software automatically calculates and reports sIgE results (quantitatively) and tIgE results (semi- quantitatively).
LIMITATIONS OF THE PROCEDURE
A definitive clinical diagnosis should only be made in conjunction with all available clinical findings by medical professionals and shall not be based on results of a single diagnostic method only.
In certain areas of application (e.g. food allergy), circulating IgE antibodies may remain undetectable although a clinical manifestation of food allergy against a certain allergen may be present, because these antibodies may be specific to allergens that are modified during industrial processing, cooking or digestion and hence do not exist on the original food for which the patient is tested.
Negative venom results only indicate undetectable levels of venom specific IgE antibodies (e.g. due to long term non-exposure) and do not preclude the existence of clinical hypersensitivity to insect stings.
In children, especially up to 2 years of age, the normal range of tIgE is lower than in adolescents and adults [7]. Therefore, it is to be expected that in a higher proportion of children younger than 2 years the total IgE-level lies below the specified detection limit. This limitation does not apply to specific IgE measurement.
EXPECTED VALUES
The close association between allergen-specific IgE antibody levels and allergic disease is well known and is described thoroughly in literature [1]. Each sensitized patient will show an individual IgE profile when tested with ALEX². The IgE response with samples from healthy non-allergic individuals will be below 0.3 kUA/l for single molecular allergens and for allergen extracts when tested with ALEX². The reference area for total IgE in adults is < 100 kU/l. Good laboratory practice recommends that each laboratory establishes its own range of expected values.
PERFORMANCE CHARACTERISTICS
The performance characteristics as well as the Summary of Safety and Performance can be found on the MADx website: https://www.madx.com/extras.
WARRANTY
The performance data were obtained using the procedure outlined in this Instructions for Use. Any change or modification in the procedure may affect the results and MacroArray Diagnostics disclaims all warranties expressed (including the implied warranty of merchantability and fitness for use) in such an event. Consequently, MacroArray Diagnostics and its local distributors shall not be liable for damages indirect or consequential in such an event.
ABBREVIATIONS
| ALEX | Allergy Xplorer |
| CCD | Cross-reactive carbohydrate determinants |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IgE | Immunoglobulin E |
| IVD | In-vitro diagnostic |
| kU/l | Kilo units per Liter |
| kUA/l | Kilo units of allergen-specific IgE per liter |
| MADx | MacroArray Diagnostics |
| REF | Reference number |
| rpm | Rounds per minute |
| sIgE | Allergen-specific IgE |
| tIgE | Total IgE |
| µl | Microliter |
ALLERGEN LIST ALEX²
Allergen extracts: Aca m, Aca s, Ach d, Ail a, All c, All s, Ama r, Amb a, Ana o, Api m, Art v, Ave s, Ber e, Bos d meat, Bos d milk, Bro p, Cam d, Can f ♂ urine, Can s, Cap a, Cap h epithelia, Cap h milk, Car c, Car i, Car p, Che a, Che q, Chi spp., Cic a, Cit s, Cla h, Clu h, Cor a pollen, Cuc p, Cup s, Cyn d, Dau c, Dol spp., Equ c milk, Equ c meat, Fag e, Fic b, Fic c, Fra e, Gad m, Gal d meat, Gal d white, Gal d yolk, Hel a, Hom g, Hor v, Jug r, Jun a, Len c, Lit s, Loc m, Lol spp., Lup a, Mac i, Man i, Mel g, Mor r, Mus a, Myt e, Ori v, Ory meat, Ory s, Ost e, Ovi a epithelia, Ovi a meat, Ovi a milk, Pan b, Pan m, Pap s, Par j, Pas n, Pec spp., Pen ch, Per a, Pers a, Pet c, Pha v, Phr c, Pim a, Pis s, Pla l, Pol d, Pop n, Pru av, Pru du, Pyr c, Raj c, Rat n, Rud spp., Sac c, Sal k, Sal s, Sco s, Sec c flour, Sec c pollen, Ses i, Sin, Sol spp., Sola l, Sol t, Sus d epithel, Sus d meat, Ten m, Thu a, Tri fo, Tri s, Tyr p, Ulm c, Urt d, Vac m, Ves v, Zea m flour
Purified natural components: nAct d 1, nApi m 1, nAra h 1, nAra h 3, nBos d 4, nBos d 5, nBos d 6, nBos d 8, nCan f 3, nCor a 9, nCor a 11, nCup a 1, nCry j 1, nEqu c 3, nFag e 2, nGad m 1, nGad m 2 + 3, nGal d 2, nGal d 3, nGal d 4, nGal d 5, nGly m 5, nGly m 6, nJug r 4, nMac i 2S Albumin, nOle e 7 (RUO), nPap s 2S Albumin, nPis v 3, nPla a 2, nTri a aA_TI
Recombinant components: rAct d 10, rAct d 2, rAct d 5, rAln g 1, rAln g 4, rAlt a 1, rAlt a 6, rAmb a 1, rAmb a 4, rAna o 2, rAna o 3, rAni s 1, rAni s 3, rApi g 1, rApi g 2, rApi g 6, rApi m 10, rAra h 2, rAra h 6, rAra h 8, rAra h 9, rAra h 15, rArg r 1, rArt v 1, rArt v 3, rAsp f 1, rAsp f 3, rAsp f 4, rAsp f 6, rBer e 1, rBet v 1, rBet v 2, rBet v 6, rBla g 1, rBla g 2, rBla g 4, rBla g 5, rBla g 9, rBlo t 10, rBlo t 21, rBlo t 5, rBos d 2, rCan f 1, rCan f 2, rCan f 4, rCan f 6, rCan f Fel d 1 like, rCan s 3, rCav p 1, rChe a 1, rCla h 8, rClu h 1, rCor a 1.0103, rCor a 1.0401, rCor a 8, rCor a 12 (RUO), rCor a 14, rCra c 6, , rCuc m 2, rCyn d 1, rCyp c 1, rDau c 1, rDer f 1, rDer f 2, rDer p 1, rDer p 10, rDer p 11, rDer p 2, rDer p 20, rDer p 21, rDer p 23, rDer p 5, rDer p 7, rEqu c 1, rEqu c 4, rFag s 1, rFel d 1, rFel d 2, rFel d 4, rFel d 7, rFra a 1 + 3, rFra e 1, rGal d 1, rGly d 2, rGly m 4, rGly m 8, rHev b 1, rHev b 3, rHev b 5, rHev b 6.02, rHev b 8, rHev b 11, rHom s LF, rJug r 1, rJug r 2, rJug r 3, rJug r 6, rLep d 2, rLol p 1, rMal d 1, rMal d 3, rMala s 11, rMala s 5, rMala s 6, rMal d 2, rMer a 1, rMes a 1 (RUO), rMus m 1, rOle e 1, rOle e 9, rOry c 1, rOry c 2, rOry c 3, rPar j 2, rPen m 1, rPen m 2, rPen m 3, rPen m 4, rPer a 7, rPhl p 1, rPhl p 12, rPhl p 2, rPhl p 5.0101, rPhl p 6, rPhl p 7, rPho d 2, rPhod s 1, rPis v 1, rPis v 2, rPis v 4 (RUO), rPla a 1, rPla a 3, rPla l 1, rPol d 5, rPru p 3, rPru p 7 (RUO), rRaj c Parvalbumin, rSal k 1, rSal s 1, rSco s 1, rSes i 1, rSin a 1, rSola l 6, rSus d 1, rThu a 1, rTri a 14, rTri a 19, rTyr p 2, rVes v 1, rVes v 5, rVit v 1, rXip g 1, rZea m 14
REFERENCES
- Hamilton, R.G.. (2008). Assessment of human allergic diseases. Clinical Immunology. 1471-1484. 10.1016/B978-0-323-04404-2.10100-9.
- Harwanegg C, Laffer S, Hiller R, Mueller MW, Kraft D, Spitzauer S, Valenta R. Microarrayed recombinant allergens for diagnosis of allergy. Clin Exp Allergy. 2003 Jan;33(1):7-13. doi: 10.1046/j.1365-2222.2003.01550.x. PMID: 12534543.
- Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchêne M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Lidholm J, Müller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen HD, Spitzauer S, Suck R, Swoboda I, Thomas W, Tinghino R, Van Hage-Hamsten M, Virtanen T, Kraft D, Müller MW, Valenta R. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002 Mar;16(3):414-6. doi: 10.1096/fj.01-0711fje. Epub 2002 Jan 14. PMID: 11790727
- Ferrer M, Sanz ML, Sastre J, Bartra J, del Cuvillo A, Montoro J, Jáuregui I, Dávila I, Mullol J, Valero A. Molecular diagnosis in allergology: application of the microarray technique. J Investig Allergol Clin Immunol. 2009;19 Suppl 1:19-24. PMID: 19476050.
- Ott H, Fölster-Holst R, Merk HF, Baron JM. Allergen microarrays: a novel tool for high-resolution IgE profiling in adults with atopic dermatitis. Eur J Dermatol. 2010 Jan-Feb;20(1):54-
61. doi: 10.1684/ejd.2010.0810. Epub 2009 Oct 2. PMID: 19801343. - Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010 Oct;40(10):1442-60. doi: 10.1111/j.1365-2222.2010.03585.x. Epub 2010 Aug 2. PMID: 20682003.
- Martins TB, Bandhauer ME, Bunker AM, Roberts WL, Hill HR. New childhood and adult reference intervals for total IgE. J Allergy Clin Immunol. 2014 Feb;133(2):589-91.
For details on the performed analytical and clinical studies refer to the performance characteristics at https://www.madx.com/extras.
CHANGE HISTORY
| Version | Description | Replaces |
| 11 | nGal d1 changed to rGal d1; URL updated to madx.com; CE supplemented with the number of the Notified Body; change history added | 10 |

© Copyright by MacroArray Diagnostics
MacroArray Diagnostics (MADx)
Lemböckgasse 59, Top 4
1230 Vienna, Austria
+43 (0)1 865 2573
www.madx.com
Version number: 02-IFU-01-EN-11 Released: 09-2024
Quick Guide
MacroArray Diagnostics
Lemböckgasse 59, Top 4
1230 Vienna
madx.com
CRN 448974 g
Documents / Resources
 |
MacroArray ALLERGY XPLORER Macro Array Diagnostics [pdf] Instructions 91201229202JQ, 02-2001-01, 02-5001-01, ALLERGY XPLORER Macro Array Diagnostics, ALLERGY XPLORER, Macro Array Diagnostics, Array Diagnostics, Diagnostics |




