1. Product Overview
The iHealth COVID-19 Antigen Rapid Test is an over-the-counter (OTC) self-test authorized by the FDA under an Emergency Use Authorization (EUA). This test is designed for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal swab specimens from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.

Figure 1: iHealth COVID-19 Antigen Rapid Test Kit Packaging
Key Features:
- FDA EUA Authorized: Authorized for emergency use to detect COVID-19.
- Rapid Results: Provides results in just 15 minutes.
- Non-invasive Nasal Swab: Easy to use with minimal discomfort, requiring only 1/2 to 3/4 inch insertion.
- At-Home Testing: Conveniently perform the test in your own home without needing to send samples to a lab.
- Ages 2 and Above: Self-administered for individuals aged 15 years and older. Adult-collection is required for testing children 2-14 years old.
- Mobile App Integration: Optional iHealth Test app available for step-by-step instructional videos and group testing management.
- Independently-Sealed Components: Swabs and test cards are individually sealed for safety and hygiene.
2. Kit Contents
Each iHealth COVID-19 Antigen Rapid Test kit contains the following components:

Figure 2: Components included in the iHealth COVID-19 Antigen Rapid Test Kit
- Test Card (individually sealed)
- Nasal Swab (individually sealed)
- Tube with Sample Extraction Solution and Dropper Tip
- Instruction for Use / Quick Start Guide
3. Preparation Before Testing
- Wash your hands thoroughly with soap and water for at least 20 seconds, then dry them completely.
- Ensure the test kit is at room temperature (59-86°F / 15-30°C) before use.
- Check the expiration date on the kit box. Do not use if expired.
- Open the kit and place all components on a clean, flat surface.
- Download the iHealth Test app (optional) for guided instructions and video tutorials.
4. Performing the Test
Follow these steps carefully to perform the iHealth COVID-19 Antigen Rapid Test:

Figure 3: Simplified 4-step process for the iHealth COVID-19 Antigen Rapid Test
- Swab Collection: Gently insert the entire soft tip of the swab into one nostril about 1/2 to 3/4 of an inch (1 to 1.5 cm). Firmly rub the swab in a circular motion around the inside of the nostril 5 times. Using the same swab, repeat the process for the other nostril.
- Sample Mixing: Immediately insert the swab into the tube containing the sample extraction solution. Swirl the swab vigorously in the liquid 15 times. While removing the swab, squeeze the sides of the tube to extract as much liquid as possible from the swab. Discard the swab.
- Apply Sample to Test Card: Place the dropper tip firmly onto the sample extraction tube. Invert the tube and gently squeeze 3 drops of the solution into the sample well (marked 'S') on the test card.
- Wait for Results: Set a timer for 15 minutes. Do not read the results before 15 minutes or after 30 minutes, as this may lead to inaccurate results.

Figure 4: Proper technique for non-invasive nasal swab collection.

Figure 5: Adult-assisted sample collection for children aged 2-14.
5. Interpreting Results
Read the results on the test card exactly 15 minutes after applying the sample. The test card has two lines: a Control Line (C) and a Test Line (T).

Figure 6: Example of a test card with clear results.
| Result | Appearance on Test Card | Interpretation |
|---|---|---|
| Positive | Both the Control Line (C) and Test Line (T) are visible. Even a faint T line indicates a positive result. | SARS-CoV-2 nucleocapsid protein antigen was detected. You are presumed to be positive for COVID-19. Isolate and seek follow-up care with your healthcare provider. |
| Negative | Only the Control Line (C) is visible. The Test Line (T) is not visible. | SARS-CoV-2 nucleocapsid protein antigen was not detected. A negative result does not rule out COVID-19. Repeat testing is recommended. |
| Invalid | The Control Line (C) is not visible, regardless of whether the Test Line (T) is visible. | The test did not work correctly. This could be due to incorrect procedure or a faulty test. Discard the test and retest with a new kit. |
For detailed guidance on interpreting results and next steps, refer to the official instructions for use or consult a healthcare professional.
6. Storage and Disposal
Storage:
- Store the test kit at room temperature (59-86°F / 15-30°C) in its original sealed pouch.
- Do not freeze the kit.
- Keep out of direct sunlight.
Disposal:
After use, place all used test components (swab, test card, and tube) in a sealed plastic bag and dispose of them in your household trash. Follow local regulations for waste disposal if specific guidelines for medical waste are provided.
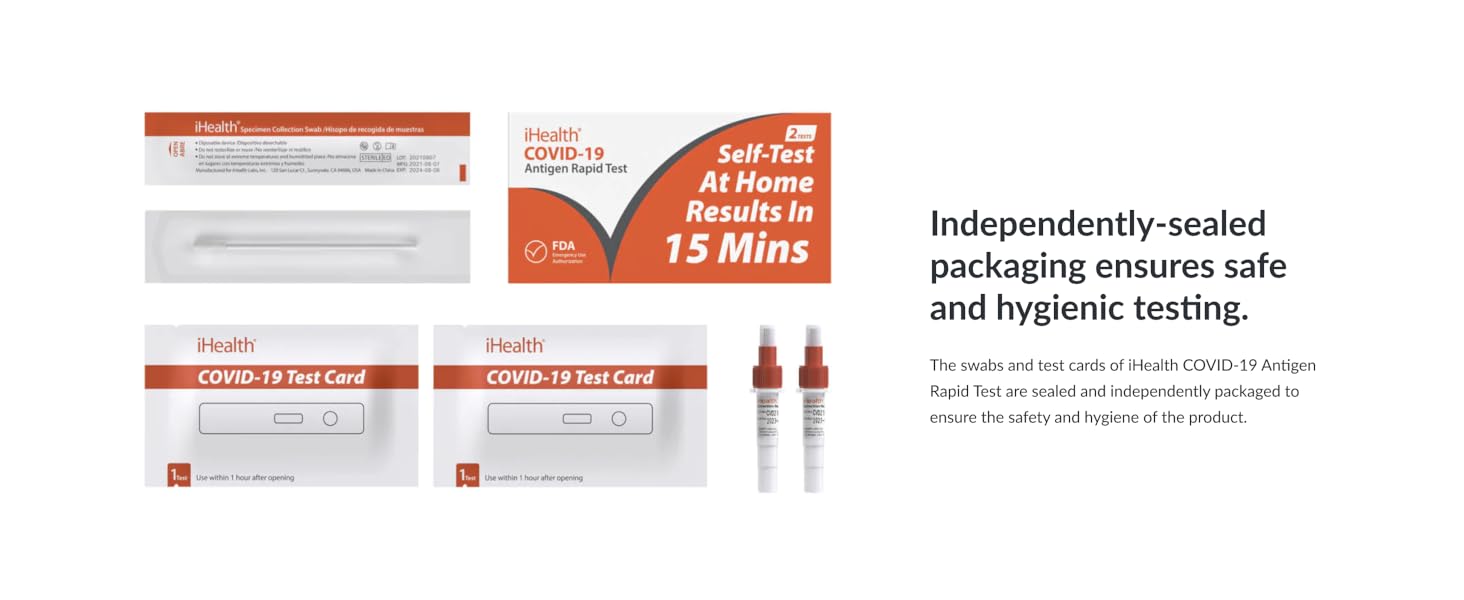
Figure 7: Independently-sealed packaging ensures product integrity and hygiene.
7. Troubleshooting
If you encounter issues during testing, refer to the following common scenarios:
| Problem | Possible Cause | Solution |
|---|---|---|
| No Control Line (C) appears. | Incorrect test procedure, insufficient sample volume, or expired/damaged test kit. | The test is invalid. Discard the current test and repeat the procedure with a new test kit, ensuring all steps are followed precisely. |
| Faint Test Line (T) appears. | Low viral load or early stage of infection. | A faint line still indicates a positive result. Follow guidelines for positive results, including isolation and consulting a healthcare provider. |
| Results are unclear or smudged. | Too much or too little sample solution, or reading the test outside the 15-30 minute window. | Discard the test and retest with a new kit, paying close attention to the number of drops and the timing for reading results. |
8. Specifications
| Attribute | Detail |
|---|---|
| Item Model Number | ICO-3000 |
| Manufacturer | iHealth |
| Package Dimensions | 6.38 x 3.66 x 1.5 inches; 3.21 ounces |
| Test Type | Antigen Rapid Test |
| Sample Type | Anterior Nasal Swab |
| Result Time | 15 minutes |
9. Important Information & Legal Disclaimer
This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA; This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
10. Warranty and Support
Warranty:
The iHealth COVID-19 Antigen Rapid Test comes with a 1-Year Limited Warranty. Please retain your proof of purchase for warranty claims.
Customer Support:
For any questions, technical assistance, or support regarding your iHealth product, please contact our US-Based Customer Support team. Refer to the contact information provided on the product packaging or the official iHealth website for the most current support details.
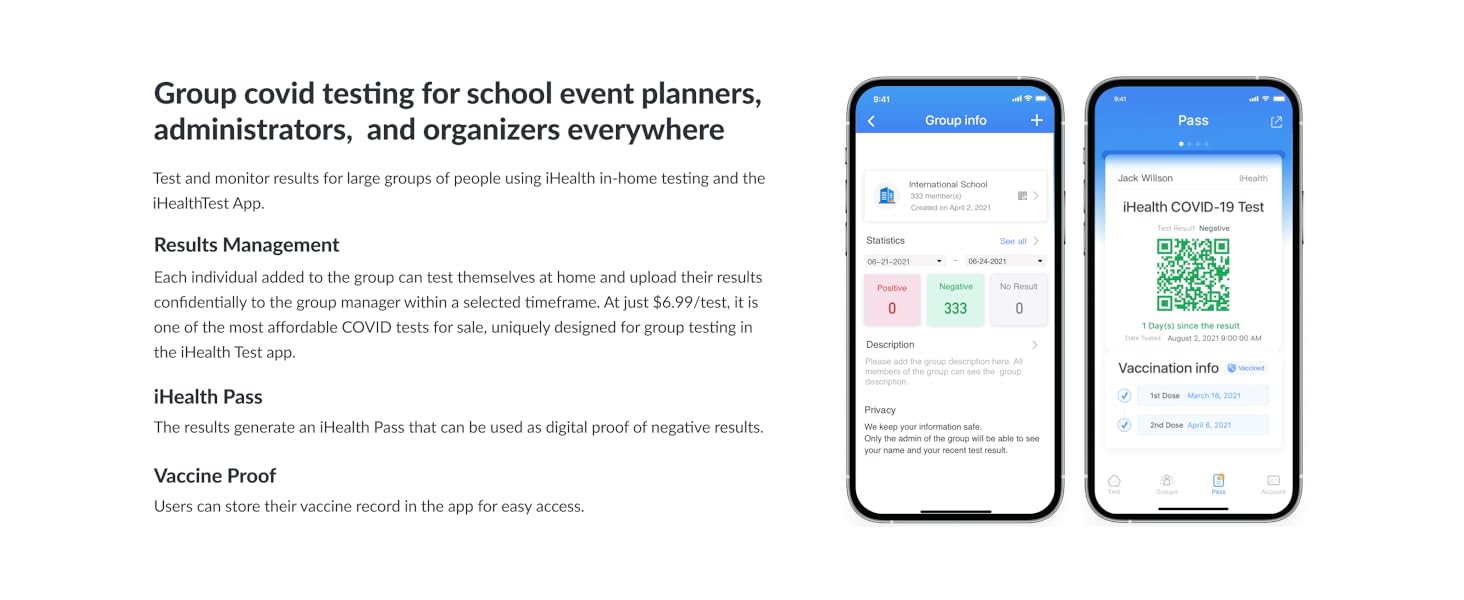
Figure 8: The iHealth Test app can assist with group testing management.





